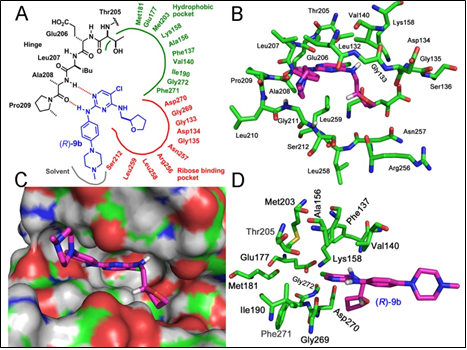
(R)-9b : A New ACK1 Inhibitor
Limited Efficacy of Existing AR-antagonists
ACK1, a crucial regulator of CRPC tumor growth
ACK1 is a structurally unique non-receptor tyrosine kinase that is upregulated in ~25% of prostate adenocarcinomas and almost 50% of CRPCs. Importantly, 10 out of 13 CRPCs exhibited 5- to >100-fold ACK1 mRNA overexpression. Moreover, expression of activated ACK1 not only correlates positively with the progression of disease to CRPC stage, but also revealed that patients that display moderate to strong staining of activated ACK1 have poor prognosis.
Mechanistic studies reveal that ACK1 tyrosine kinase interacts with AR, causing its phosphorylation and acetylation. The modified AR interacts with ACK1, and ACK1/AR complex deposit novel epigenetic marks, histone H4 Tyr88-phosphorylation (pY88-H4) directly upstream of AR gene locus, upregulating its expression.
ACK1 inhibitor, (R)-9b erased pY88-H4 marks causing global loss of AR and AR-V7 expressing, significantly compromising Enz/Abi-resistant tumor growth.
(R)-9b, a first-in-class `dual' inhibitor with Immune modulatory activity
Significantly, (R)-9b exhibited an unique activity- it activated CD8+ T cells in tumor microenvironment, causing robust immune response against tumors. This novel immune modulatory activity makes this compound a rare `dual' inhibitor that not only suppresses AR expression in tumor cells but also activate effector T cells to target cancer cells.
Prostate tumors exhibit ICB (immune checkpoint blockade) resistance. (R)-9b was able to activate T cells isolated from blood of Abiraterone and Enzalutamide treated CRPC patients. This is a significant data to indicate a new strategy to activate immune cells in patients with prostate cancer and sensitize them to ICB therapy.
(R)-9b, Oral inhibitor: IND & Phase I Clinical Trial (PHAROS)

